🎧Renishaw AM & metrology provides flexibility for medical manufacturer
🎧Renishaw AM & metrology provides flexibility for medical manufacturer
Croom Precision Medical has selected Renishaw as its partner for additive manufacturing (AM) and metrology. It will use Renishaw technology throughout the production and validation of its ISO: 13485 certified medical devices.
The company has found that using Renishaw’s AM technology enables them to incorporate complex features into their implant designs at a commercially viable cost. They also note that Renishaw’s integrated AM software, combined with its metrology solutions, helps them maintain traceability throughout their manufacturing process, which is vital in a heavily regulated environment.

Background
Croom Precision Medical (CPM) is at the forefront of Ireland’s thriving medical device industry. Established in 1984, CPM has supplied Class I, II, and III medical devices to the orthopaedic market for over 35 years. Since these devices ultimately impact people’s lives, it is crucial to implement rigorous quality procedures to ensure their safety and effectiveness during and after implantation.
“We strive to achieve consistency and reliability in our manufactured products. CPM envisions becoming a centre of excellence for additive manufacturing in Ireland,” explains Patrick Byrnes, Research and Development Manager at CPM. CPM operates an integrated management system (IMS) that encompasses ISO: 9001, ISO: 13485, and ISO: 14001 accreditation. To demonstrate compliance with these standards, CPM must ensure transparency and traceability at every stage of the manufacturing process.
Medical implant designs are rapidly advancing. Manufacturers are increasingly integrating novel and complex structures into these designs to enhance performance and longevity. However, creating such structures with traditional manufacturing techniques can be challenging and may not always be economically feasible.
To evaluate the quality of parts, CPM conducts a series of offline tests to assess the implants’ chemical, mechanical, and morphological properties. While these tests are essential for measuring the mechanical and chemical consistency of the implants, they can be time-consuming and can raise the overall cost per part. CPM’s goal is to transform production so that more monitoring occurs during manufacturing. “We’re looking for a red or green light, indicating whether the part is good or bad. Currently, we perform extensive offline testing, aiming to minimise it to reduce our cost of goods. We have made substantial progress in that direction over the past couple of years; there’s still some work to be done, but we believe we are ahead of many of our competitors in the area of in-process monitoring,” Byrnes noted.
Solution
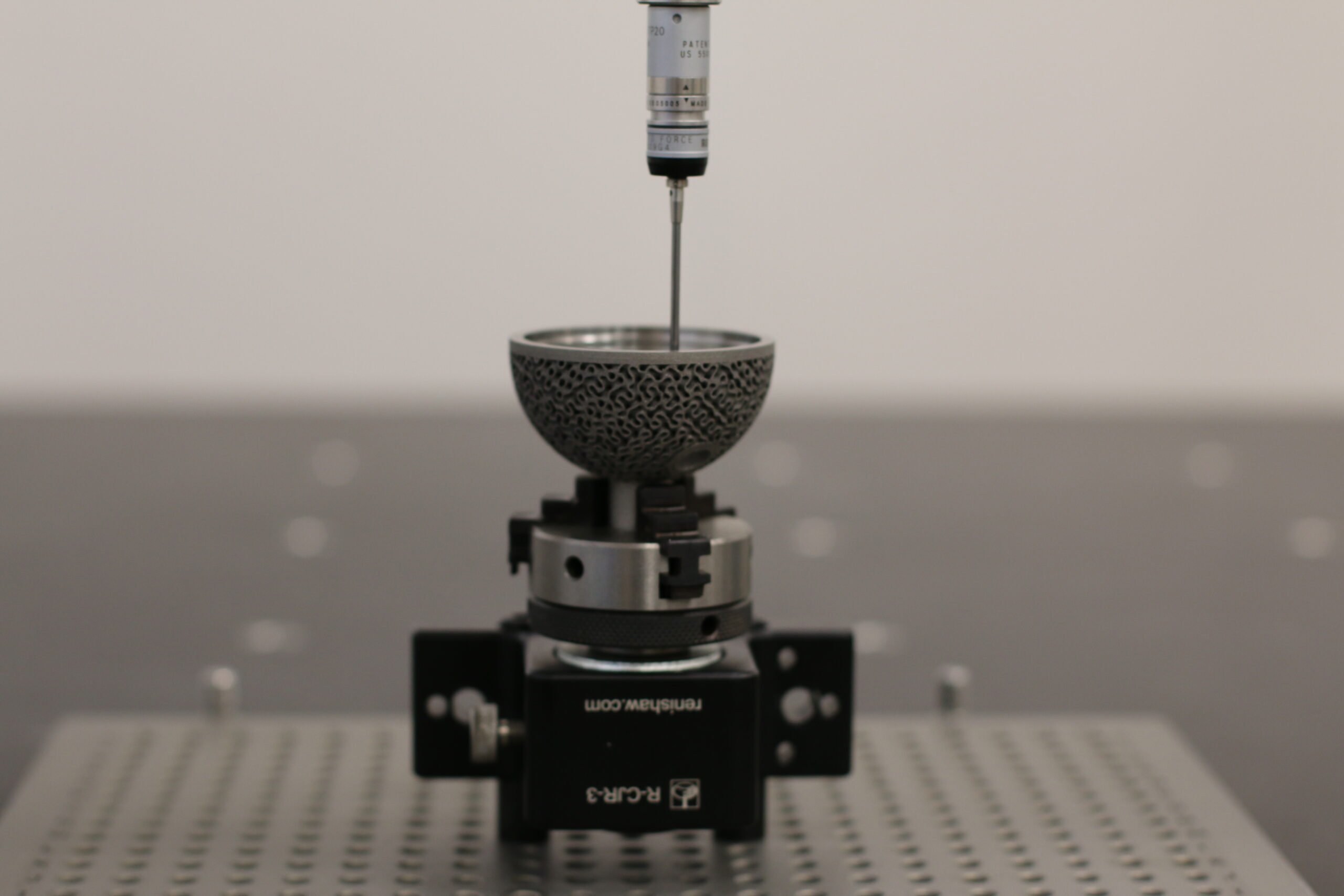
Emerging from the aerospace sector, Renishaw has a proven history of providing solutions that enhance manufacturing efficiency and part safety in highly regulated environments. CPM is applying Renishaw’s technology in the medical sector for the same purpose. CPM has invested in a RenAM 500M additive manufacturing system, which it uses to 3D print medical devices in titanium. Renishaw’s AM technology can create complex structures in a single manufacturing operation. For instance, CPM has begun to evaluate the use of gyroid lattices on acetabular cups, a design feature that was previously not economically feasible before their investment in AM. The resulting implants have undergone rigorous quality testing, and CPM reports notably good density and Young’s Modulus values.
In addition to the advanced manufacturing capabilities of AM, Byrnes emphasises that inline process monitoring is what truly sets apart Renishaw’s technology and provides CPM with a competitive advantage: “The Renishaw software equips us with all the necessary information to ensure the quality of our parts, from build reports to post-processing, enabling us to trace our parts from beginning to end.” To promote quality and consistency, CPM has also selected Renishaw as its partner for precision metrology equipment, employing Renishaw technology at various stages of their testing and part validation. The team has long recognised Renishaw’s reputation for producing reliable, robust metrology equipment: “When you visit multinational companies and see all the Renishaw products on the shop floor, they have a reputation for being repeatable, reliable, and capable of consistently operating in harsh environments,” commented Byrnes. CPM exemplifies how Renishaw can provide a comprehensive solution with technology that supports the manufacturing of technically ambitious parts and quality verification.

Results
CPM reports an increased market share, directly attributed to its investment in AM technology. “We have captured new market share in international markets, specifically in East Asia and North America, which were previously unattainable for us. Only in the last two years have these markets become accessible. We chose Renishaw as a development partner, not just as a supplier, envisioning the next few years as a strategic partnership to grow our additive manufacturing base here in Ireland.”
Healthcare stands out as one of the leading industries for AM development. Although significant progress has already been made, ample room for growth still exists. CPM views Renishaw as a strategic partner in advancing AM technology. Byrnes adds, “Approaching AM for us has been a journey; it’s an entirely different way of thinking. It’s a more laboratory-like environment, a more cognitive approach to the process as opposed to conventional engineering.”
In the coming years, CPM will continue to leverage the knowledge gained from its rigorous development programs and collaborate closely with customers and regulatory bodies to advance additive manufacturing technology and support enhancements in healthcare.